Which Is the Best Example of Decreasing Entropy
1 and the free expansion of a gas see Ex. Decreasing entropy is disrupted usually by a person or something living.

Entropy And The Second Law Of Thermodynamics Disorder And The Unavailability Of Energy Physics
Without dissipating heat somewhere in the surrounding or nearby matter or environment causes a useful rise in the temperature of air about 600 degree centigrade which.

. This defies the second law of thermodynamics. B Cells require a constant input of energy to maintain their high level of organization. An increasing entropy is something that is left to be like the other answers.
C Conversion of energy from one form to another is always accompanied by some gain of free energy. The trivial everyday phenomenon of something cooling down is the prototypical example of entropy decreasing. Hence randomness is decreasing here.
State the second law of thermodynamics in terms of energy flow and in terms of degradation or distribution of energy. 1 mol of helium gas at 273K and 20L. 12 mol of liquid helium at 100K.
However to the best of our knowledge there is no up-to-date systematic and comprehensive review on the applications of entropy in cryptography and related areas. Which process is an example of entropy decreasing. 1 mol of helium gas at 273K and 40L.
As another example an information. A dining room is cleaned to remove dust and thus the disorder is decreasing. A quantity of water has more entropy when it is.
But because at all times the tea was warmer than the room the. C Dry ice placed in an open vessel. For example plants use energy from the sun in tiny energy factories called chloroplasts.
Now of course as the tea cooled the room warmed. Reactions that have a positive delta G typically have a decrease in entropy. But because at all times the tea was warmer than the room the.
Its as simple as that. A pile of dirt on the floor is cleaned up with a broom. Solid carbon dioxide will turn into gaseous carbon dioxide.
Spontaneous processes are those which increase entropy. The smaller the volume the less the molecules can move around which also reduces the number of possible different states and hence less the entropy. In both of these the total entropy increases though that of parts of the system may decrease.
Its as simple as that. The trivial everyday phenomenon of something cooling down is the prototypical example of entropy decreasing. A Every energy transformation by a cell decreases the entropy of the universe.
12 mol of helium gas at 100K and 20L. Now of course as the tea cooled the room warmed. Freezing an ice cube if you follow the entropy equation which I dont have with me is one example of this.
Cells in a body coming together. Which of the following has the most entropy individual nucleotides or a nucleic acid. Size of air is decreased without removing heat from the air while Enthalpy remains same this decrease in Entropy without decreasing Enthalpy ie.
For example the Big Freeze theory states the Universe will eventually reach maximum entropy whereby energy reaches a state of disorder that makes it unusable for work or information storage. Which is the best example of decreasing entropy. 4In a colder gas the molecules or atoms are not so energetic and so do not occupy so many different energetic states and hence will have less entropy.
Examples of spontaneous processes are the flow of heat from a hotter to a colder body see Ex. Entropy often comes up in theories about the ultimate fate of the Universe. Steam condenses to liquid water.
Solid to liquid transformation. For example a method for decreasing the entropy of the information leaked from side channels was introduced by Dhavlle et al. 12 mol of helium gas at 273K and 20L.
Nalin 4 1 year ago. Using chlorophyll in the process called photosynthesis they convert the suns energy into storable form. Similarly the sun heating up a patch of earth makes the entropy of the sun decrease the entropy of the earth increase and the entropy of the solar system increase.
In this process Entropy is decreased ie. D Without an input of energy organisms would tend toward decreasing entropy. Solve any question of Chemical Thermodynamics with-.
D Melting of ice. Such a state is cold uniform and sparse with all things stopped. A deserted field becomes overgrown with weeds and thus the entropy of the field increases.
This is completely general. For example my question is Rank these systems in order of decreasing entropy The options are. The cost of this local decrease in entropy is a universal increase in entropy from the.
Which organelle is the storehouse for most of a cells genetic information. 1 mol of hydrogen gas at 273K and 40L. Which is an example of decreasing entropy in a closed system.
Order can be produced with an expenditure of energy and the order associated with life on the earth is produced with the aid of energy from the sun. The entropy of the glass increases the entropy of the room decreases and the entropy of the combined system increases dS dQ 273 K - dQ 293 K. A cyclist pedals harder while riding uphill and thus energy is supplied and the entropy decreases.
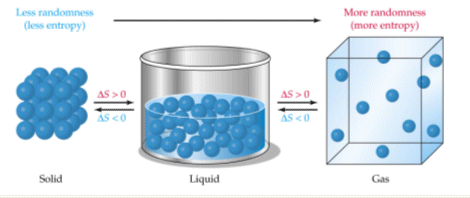
Which Of The Following Processes Shows A Decrease In Entropy Of The System Socratic

Entropy And The Second Law Of Thermodynamics Disorder And The Unavailability Of Energy Physics

Thermodynamics Does Entropy Increase With A Decrease Or With An Increase In A System S Temperature Physics Stack Exchange
No comments for "Which Is the Best Example of Decreasing Entropy"
Post a Comment